Services
We are together with you every step of the way – in ensuring usability and your compliance is always our top priority.
Key Services
GxP Risk Assessment & Benchmarking
Support of inspection readiness and post audit response preparation
Life Science Software Validation & Implementation
Development & Implementation of Quality Systems
Training & Coaching
Support in Remediation plans and CAPAs
Due Diligence in M & A
Performing Internal Inspections to Ensure All Time Readiness and Compliance
Building Culture of Quality
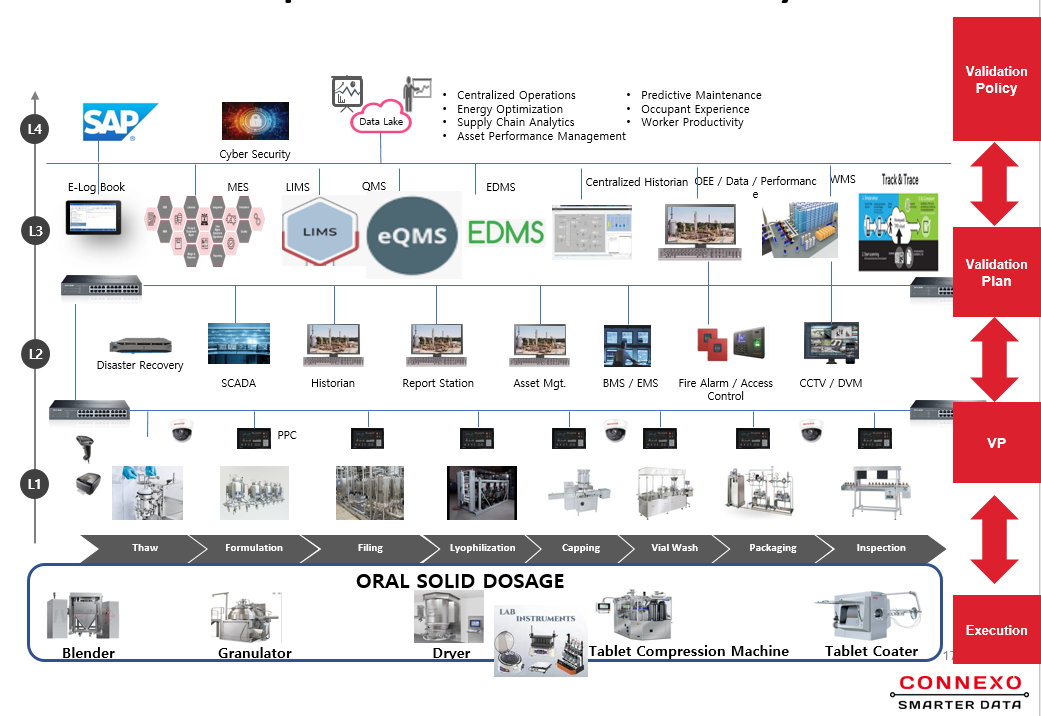
EQMS (EDMS)
Manufacturing Execution System (MES)
Electronic Batch Manufacturing Record(EBMR)
Distributed Control Systems (DCS)
PLCs & SCADA
Environmental Monitoring Systems
Electronic Log Books
Access Control Systems
Learning Management Systems (LMS)
Warehouse Management System
Track and Trace
Barcode Scanners & Barcode Labels
GxP Audit Remediation
CSV & CSA for all GxP Systems
GxP Training
GxP Risk Assessment & Gap Analysis
Version Upgrades and Patch Management
AI Based FDA Observation Management
Data Analytics & Visualisation in line with USFDA Process Metrics Guide
Risk Based User Access Management for GxP Systems
Risk based implementations
We are together with you every step of the way – in ensuring usability and your compliance is always our top priority.